Seminarios Semanales
Área Bioquímica y Biofísica Molecular.
Lunes 10:30 hs; Sala de Reuniones Depto.Química
13-06-2016
Disertante: Biól. Iván Felsztyna
Insecticidal 3-benzamido-N-phenylbenzamides specifically bind with high affinity to a novel allosteric site in housefly GABA receptors. Ozoe et al., Pesticide Biochemistry and Physiology 107, 285-292, 2013.
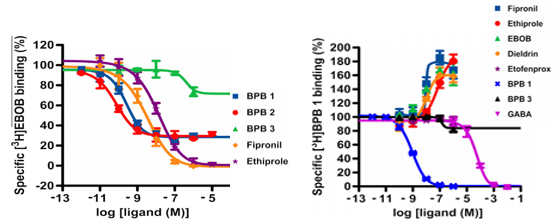
γ-Aminobutyric acid (GABA) receptors (GABARs) are an important target for existing insecticides such as fiproles. These insecticides act as noncompetitive antagonists (channel blockers) for insect GABARs by binding to a site within the intrinsic channel of the GABAR. Recently, a novel class of insecticides, 3-benzamido-N-phenylbenzamides (BPBs), was shown to inhibit GABARs by binding to a site distinct from the
site for fiproles. We examined the binding site of BPBs in the adult housefly by means of radioligand binding and electrophysiological experiments. 3-Benzamido-N-(2,6-dimethyl-4-perfluoroisopropylphenyl)-2 fluorobenzamide (BPB1) (the N-demethyl BPB) was a partial, but potent, inhibitor of [3H] 40-ethynyl-4-n propylbicycloorthobenzoate (GABA channel blocker) binding to housefly head membranes, whereas the 3-(N-methyl) benzamido congener (the N-methyl BPB) had low or little activity. A total of 15 BPB analogs were tested for their abilities to inhibit [3H]BPB1 binding to the head membranes. The N-demethyl analogs, known to be highly effective insecticides, potently inhibited the [3H]BPB1 binding, but the N-methyl analogs did not even though they, too, are considered highly effective. [3H]BPB1 equally bound to the head membranes from wild-type and dieldrin-resistant (rdl mutant) houseflies. GABA allosterically inhibited [3H]BPB1 binding. By contrast, channel blocker-type antagonists enhanced [3H] BPB1binding to housefly head membranes by increasing the affinity of BPB1. Antiparasitic macrolides, such as ivermectin B1a, were potent inhibitors of [3H]BPB1 binding. BPB1inhibited GABA-induced currents in housefly GABARs expressed in Xenopus oocytes, whereas it failed to inhibit L-glutamate induced currents in inhibitory L-glutamate receptors. Overall, these findings indicate that BPBs act at a novel allosteric site that is different from the site for channel blocker- type antagonists and that is probably overlapped with the site for macrolides in insect GABARs.